Abstract
Similar Risk of Relapse and Survival with Post-Transplant Cyclophosphamide Compared to Standard GVHD Prophylaxis after Allogeneic Hematopoietic Cell Transplantation for AML and MDS
Background: Post-transplant cyclophosphamide (PTCy) is frequently used after allogeneic hematopoietic cell transplant (HCT) as it results in lower rates of graft versus host disease (GVHD). In addition to its predominant use in haploidentical and HLA mismatched donor HCT, PTCy showed potential to become the standard of care GVHD prophylaxis in a setting of HLA matched related and unrelated donor HCT as well (Bolaños-Meade, Lancet 2019). However, recent registry study reported higher risk of relapse after PTCy-based vs.calcineurin inhibitor (CNI) and methotrexate (MTX) GVHD prophylaxis in patients with acute myeloid leukemia (AML) receiving matched sibling donor HCT (Nagler, Transplant Cell Ther 2022). Thus, additional studies are needed to determine whether the benefit of GVHD prevention with PTCy comes at the cost of compromising the graft versus leukemia effect yielding higher relapse of AML and myelodysplastic syndromes (MDS) after HCT. In this retrospective study, we compared the HCT outcomes of PTCy-based vs. CNI-based GVHD prophylaxis in patients with AML and MDS.
Methods: We included patients with AML and MDS who received an allogeneic HCT in first or second complete remission at our institution from 1/1/2014 to 12/31/2019. Patients received either a PTCy-based or a CNI-based GVHD prophylaxis regimen. CNI-based regimen consisted of either CNI/sirolimus +/- other (CNI/Siro group) or CNI/MTX +/- other (CNI/MTX group). The graft source in all the patients was peripheral blood (n=582) or bone marrow (n=23).
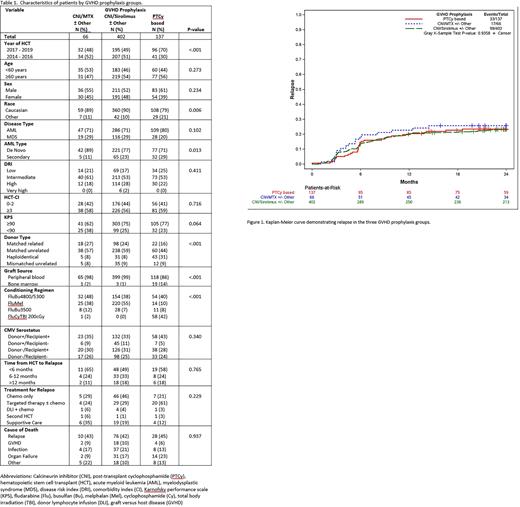
Results: Total of 605 patients were identified: 442 with AML and 163 with MDS (Table 1.). GVHD prophylaxis with PTCy was used in 137 (23%), CNI/Siro in 402 (66%) and CNI/MTX in 66 (11%) patients. The PTCy group had higher proportion of patients who were Caucasian, received haploidentical donor HCT, reduced intensity (RIC) or non-myeloablative (NMA) conditioning or a bone marrow graft. Otherwise, baseline characteristics were similar between the GVHD prophylaxis groups. With a median follow up of 47 months, 149 (25%) patients relapsed, and 265 (44%) patients died. The probability of relapse at 2 years after HCT was similar between the GVHD prophylaxis groups: 23% in PTCy, 26% in CNI/Siro and 23% in CNI/MTX (p=0.94) (Figure 1). Similarly, there were no significant differences in 2-year overall survival (OS) between the groups: 58% in PTCy, 62% in CNI/Siro and 69% in CNI/MTX (p=0.33). We also observed no differences in non-relapsed mortality (NRM) between the groups.
Median time to post-HCT relapse was 175 days in PTCy group, 180 days in CNI/Siro group and 131 days in CNI/MTX group. Management for post-HCT relapse included chemotherapy alone (n=58), chemotherapy +/- targeted therapy (n=53), chemotherapy + donor lymphocyte infusion (n=6), second HCT (n=3) and supportive care (n=29). GVHD prophylaxis groups did not differ by type of treatment used for relapse. Post-relapse OS at 2 years was 6% in PTCy, 20% in CNI/Siro and 31% in CNI/MTX groups (p=0.34).
In multivariable analysis, the type of GVHD prophylaxis regimen did not significantly influence the post-HCT relapse, NRM, OS or post-relapse survival. Relapse was affected by DRI (p<0.001) and by type of conditioning regimen (p=0.002). NRM was influenced by HCT-CI (p=0.007) and by type of conditioning regimen (p=0.006). OS was inferior with high/very high risk DRI (p<0.001) and HCT-CI ≥ 3 (p<0.001). Survival after post-HCT relapse was better in patients relapsing >12 months after receiving HCT (p=0.003) and in those receiving treatment other than supportive care (p<0.001).
Conclusion: In patients with AML and MDS receiving allogeneic HCT, we found similar risk of relapse and survival with PTCy-based and standard GVHD prophylaxis regimens. Survival after post-HCT relapse was also similar between the GVHD prophylaxis groups. The findings of this study support the use of PTCy-based regimen as GVHD prophylaxis after allogeneic HCT in patients with AML and MDS.
Disclosures
Faramand:Kite/Gilead: Research Funding; Novartis: Research Funding. Lazaryan:Humanigen: Consultancy; AvroBio: Consultancy; Sanofi: Consultancy; AmWel: Current equity holder in publicly-traded company; Teladoc: Current equity holder in publicly-traded company. Pidala:Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Takeda: Research Funding; Johnson and Johnson: Research Funding; Pharmacyclcis: Research Funding; Abbvie: Research Funding; BMS: Research Funding. Bejanyan:Medexus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; CareDX Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.